The Silver Chloride Formula Revealed
Welcome to a deep dive into the fascinating world of chemistry, where we explore the intriguing compound known as silver chloride. This compound, with its unique properties and applications, has been a subject of interest for scientists and industries alike. In this article, we will uncover the silver chloride formula, its composition, and its wide-ranging uses in various fields. Get ready to embark on a journey that will shed light on this versatile substance and its significant role in modern technology and beyond.
The Silver Chloride Formula: Unveiling its Chemical Composition

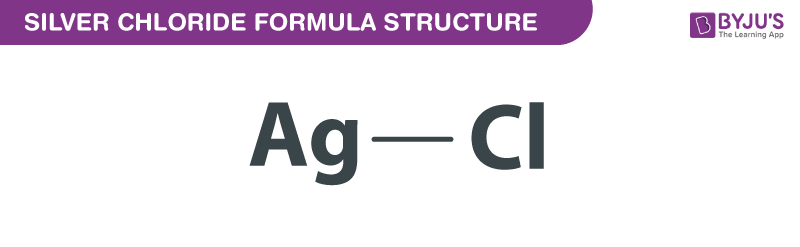
Silver chloride, often denoted as AgCl, is a white crystalline solid that has captivated the attention of chemists and researchers. Its chemical formula, AgCl, reveals the simple yet essential composition of this compound. Let’s delve into the atomic structure and unique characteristics that make silver chloride a standout in the world of chemistry.
At the heart of silver chloride's composition is the silver atom, represented by Ag, and the chlorine atom, denoted as Cl. These two elements combine in a 1:1 ratio to form the compound. The atomic weight of silver, approximately 107.87 u, and that of chlorine, around 35.45 u, contribute to the overall molecular weight of silver chloride, which is approximately 143.32 u. This precise ratio and the specific properties of silver and chlorine atoms result in the unique characteristics of silver chloride.
One of the most notable aspects of silver chloride is its high solubility in certain solvents, particularly when exposed to light. This property, known as photo-induced dissolution, is a fascinating phenomenon that has led to numerous applications in fields like photography and environmental science. When silver chloride is exposed to light, it undergoes a transformation, becoming more soluble and breaking down into its constituent elements, silver and chlorine ions.
The Chemical Reaction: Unraveling the Process
The chemical reaction that occurs when silver chloride is exposed to light can be represented as follows:
$AgCl \overset{\text{light}}{\longrightarrow} Ag^+ + Cl^-$
In this reaction, silver chloride (AgCl) is transformed into silver cations (Ag+) and chloride anions (Cl-). This photo-induced dissolution is a complex process involving the absorption of light energy, which excites the electrons in the silver chloride molecule, leading to its breakdown.
Furthermore, the solubility of silver chloride varies depending on the solvent and temperature. In aqueous solutions, silver chloride is only slightly soluble, with a solubility product constant (Ksp) of approximately 1.8 x 10-10 at room temperature. However, in the presence of light, this solubility can increase significantly, making silver chloride a highly useful compound in various applications.
Silver chloride's unique properties and its behavior under different conditions make it a subject of extensive research and development. Scientists and engineers continue to explore its potential, leading to innovative uses in diverse industries. From its historical role in photography to its modern applications in sensors and medical devices, silver chloride's impact is far-reaching.
Applications of Silver Chloride: A Multifaceted Compound

The versatility of silver chloride is truly remarkable, as it finds applications in a wide range of industries and fields. Let’s explore some of the key areas where silver chloride plays a crucial role:
Photography: Capturing Moments with Silver Chloride
One of the most well-known applications of silver chloride is its historical and ongoing use in photography. In the early days of photography, silver chloride was a key component in the creation of photographic emulsions. When exposed to light, silver chloride undergoes a chemical change, forming silver metal, which then darkens the photographic material. This process, known as photographic development, allowed for the capture of images and the creation of photographs.
Even in the digital age, silver chloride continues to find use in certain photographic processes. For example, in the production of black-and-white film, silver chloride is often employed as a light-sensitive material. When the film is exposed to light, the silver chloride grains undergo a series of chemical reactions, resulting in the formation of a latent image. This image is then developed and fixed, producing a tangible photograph.
The unique properties of silver chloride, such as its high sensitivity to light and its ability to form stable silver images, make it an ideal choice for various photographic applications. Its historical significance and continued relevance in photography showcase the enduring impact of this remarkable compound.
Sensors and Electronics: Detecting and Responding
Silver chloride’s sensitivity to light and its ability to undergo chemical changes make it an excellent candidate for use in sensors and electronic devices. In recent years, researchers have explored the potential of silver chloride in developing innovative sensor technologies.
One notable application is in the field of optical sensors. Silver chloride-based optical sensors can detect and measure various physical and chemical parameters, such as temperature, pressure, and humidity. By harnessing the unique photo-induced dissolution properties of silver chloride, these sensors can provide accurate and real-time data, making them valuable tools in various industries.
Additionally, silver chloride is used in conductive inks and pastes, which are employed in the manufacturing of flexible electronics and printed circuit boards. The conductivity of silver chloride, combined with its ease of processing, makes it an attractive material for these applications. This opens up new possibilities for the development of flexible and wearable electronic devices.
The versatility of silver chloride in sensor and electronic applications showcases its potential for future technological advancements and its role in shaping the next generation of devices.
Medical Devices: A Safe and Effective Choice
In the medical field, silver chloride has proven to be a valuable compound, particularly in the development of medical devices and implants. Its unique properties, such as biocompatibility and antimicrobial activity, make it an ideal material for various applications.
One notable use of silver chloride is in cardiac pacemakers. Silver chloride-based electrodes are often employed in these devices, as they provide excellent electrical conductivity and biocompatibility. The stability and reliability of silver chloride electrodes ensure the safe and effective functioning of pacemakers, which are critical for maintaining proper heart rhythm.
Furthermore, silver chloride is used in medical sensors and implants, where its antimicrobial properties are advantageous. Silver ions released from silver chloride can inhibit the growth of bacteria and other microorganisms, reducing the risk of infections associated with medical devices. This makes silver chloride an attractive choice for implants and sensors that come into direct contact with the human body.
The medical field's reliance on silver chloride underscores its importance in ensuring patient safety and the effective functioning of medical devices.
Environmental Applications: Silver Chloride’s Impact
Beyond its uses in photography, sensors, and medicine, silver chloride plays a crucial role in various environmental applications. Its unique properties and behavior make it a valuable tool for addressing environmental challenges and promoting sustainability.
Water Treatment: Ensuring Clean and Safe Water
One of the most significant environmental applications of silver chloride is in water treatment. Silver chloride compounds, such as silver chloride colloids, are employed in water purification processes due to their antimicrobial properties. Silver ions released from silver chloride can effectively inhibit the growth of bacteria, viruses, and other microorganisms, making water safe for consumption and use.
In addition to its antimicrobial activity, silver chloride's ability to adsorb certain pollutants makes it useful in removing contaminants from water. This property has led to the development of advanced water treatment technologies that utilize silver chloride-based materials, ensuring the production of clean and safe drinking water.
Air Quality Monitoring: Detecting and Mitigating Pollutants
Silver chloride’s sensitivity to light and its ability to undergo photo-induced dissolution have found applications in air quality monitoring. Silver chloride-based sensors can detect and measure the presence of various air pollutants, such as nitrogen dioxide and sulfur dioxide. These sensors provide real-time data on air quality, allowing for the implementation of effective mitigation strategies.
By utilizing silver chloride's unique properties, researchers and environmental scientists can develop advanced air quality monitoring systems. These systems not only detect pollutants but also provide insights into their sources and potential impacts, aiding in the development of sustainable and healthy environments.
Environmental Remediation: Addressing Contamination
Silver chloride’s antimicrobial and adsorptive properties make it a valuable tool in environmental remediation efforts. In situations where soil or groundwater is contaminated with pollutants, silver chloride compounds can be employed to mitigate the impact of these contaminants.
Silver chloride's ability to adsorb pollutants and its antimicrobial activity make it effective in treating contaminated sites. By using silver chloride-based materials, researchers and environmental engineers can develop sustainable and eco-friendly remediation strategies, ensuring the restoration of affected ecosystems.
The environmental applications of silver chloride showcase its potential in addressing pressing global challenges and promoting a more sustainable future.
Future Prospects: Unlocking New Possibilities
As research and development in various fields continue to advance, the future prospects of silver chloride are incredibly promising. Scientists and engineers are exploring new ways to harness the unique properties of silver chloride, leading to innovative applications and technologies.
Advanced Materials: Pushing the Boundaries
In the field of materials science, silver chloride is being investigated for its potential in the development of advanced materials. Its conductivity, sensitivity to light, and ability to undergo photo-induced dissolution make it an attractive candidate for various applications.
Researchers are exploring the use of silver chloride in the creation of smart materials that can respond to external stimuli, such as light or temperature changes. These materials could have applications in flexible electronics, wearable devices, and even in the development of self-cleaning surfaces.
Furthermore, silver chloride's unique optical properties are being studied for their potential in photonic devices and optical computing. The ability of silver chloride to interact with light in specific ways could lead to the development of advanced optical technologies, opening up new possibilities in information processing and communication.
Sustainable Technologies: A Green Future
With a growing focus on sustainability and environmental responsibility, silver chloride’s applications in sustainable technologies are gaining prominence. Its use in water treatment, air quality monitoring, and environmental remediation showcases its potential in promoting a greener and more sustainable future.
Researchers are exploring ways to further optimize silver chloride's antimicrobial and adsorptive properties for more efficient and eco-friendly applications. Additionally, the development of silver chloride-based solar cells is an area of active research, as these cells have the potential to be more efficient and cost-effective than traditional solar technologies.
The future of silver chloride in sustainable technologies holds immense promise, as it could play a crucial role in addressing environmental challenges and reducing our carbon footprint.
Medical Advancements: Enhancing Patient Care
In the medical field, silver chloride’s biocompatibility and antimicrobial properties continue to drive research and development. Scientists are exploring new ways to utilize silver chloride in the development of advanced medical devices and implants.
For example, researchers are investigating the use of silver chloride coatings on medical implants to enhance their biocompatibility and reduce the risk of infections. Additionally, silver chloride-based materials are being studied for their potential in drug delivery systems, where they could provide controlled and targeted release of medications, improving patient outcomes.
The future of silver chloride in medicine is bright, as it has the potential to revolutionize various aspects of patient care and treatment.
What is the main use of silver chloride in photography?
+Silver chloride is a light-sensitive compound used in the early days of photography to create photographic emulsions. When exposed to light, it undergoes a chemical change, forming silver metal, which darkens the photographic material, capturing an image.
How does silver chloride work in water treatment processes?
+Silver chloride’s antimicrobial properties make it effective in water treatment. Silver ions released from silver chloride can inhibit the growth of bacteria and other microorganisms, making water safe for consumption and use.
What are the advantages of using silver chloride in medical devices?
+Silver chloride is biocompatible and has antimicrobial properties, making it ideal for use in medical devices such as cardiac pacemakers and implants. Its stability and reliability ensure the safe and effective functioning of these devices.



