Unlocking the Secrets of Expanded Octet
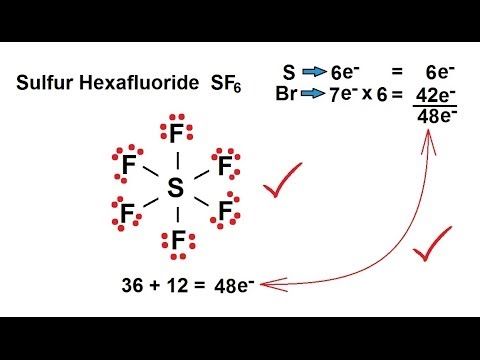
The concept of an expanded octet, a term often encountered in the field of chemistry, is a fascinating phenomenon that challenges the traditional understanding of atomic structure. This unique occurrence, which allows an atom to accommodate more than eight electrons in its outermost energy level, has intrigued scientists and researchers for decades. By delving into the intricacies of this phenomenon, we can unlock valuable insights into the behavior of atoms and molecules, shedding light on the diverse chemical reactions that shape our world.
The Expanded Octet: Breaking the Rules of Atomic Structure

In the realm of atomic theory, the concept of the octet rule is a fundamental principle. This rule suggests that atoms strive to achieve a stable electronic configuration, resembling that of the nearest noble gas, by either gaining, losing, or sharing electrons. This stability is often achieved when an atom has eight electrons in its outermost energy level, or valence shell, leading to the well-known phrase, “the octet rule.”
However, nature is full of surprises, and the expanded octet phenomenon is a testament to this. Certain elements, under specific conditions, defy the octet rule and accommodate more than eight electrons in their valence shell. This unique behavior challenges the conventional understanding of atomic stability and opens up a realm of possibilities in the world of chemical reactions.
The Intriguing Chemistry Behind Expanded Octets

The chemistry behind expanded octets is a complex interplay of atomic orbitals and electron configurations. Atoms, in their quest for stability, follow the Aufbau principle, which dictates that electrons fill the lowest energy orbitals first. However, in certain situations, this principle can be overridden by the need for atoms to form chemical bonds and attain stability.
For instance, consider the element phosphorus (P). Phosphorus typically follows the octet rule, with its five valence electrons filling the 3s and 3p orbitals. However, when phosphorus forms compounds with highly electronegative elements, such as chlorine (Cl), it can accommodate more than eight electrons in its valence shell. In the compound phosphorus trichloride (PCl3), phosphorus forms a bond with three chlorine atoms, resulting in an expanded octet configuration.
The expanded octet configuration is not limited to phosphorus; other elements, such as sulfur (S) and silicon (Si), can also exhibit this behavior. In sulfur dioxide (SO2), sulfur forms double bonds with two oxygen atoms, leading to an expanded octet configuration. Similarly, in silicon tetrachloride (SiCl4), silicon accommodates four chlorine atoms in its valence shell, once again breaking the traditional octet rule.
Understanding the Electronic Structure of Expanded Octets
To comprehend the electronic structure of expanded octets, we must delve into the realm of molecular orbitals. Molecular orbitals are formed when atomic orbitals combine to create a new set of orbitals that can accommodate a larger number of electrons. In the case of expanded octets, the valence orbitals of the central atom (such as phosphorus or sulfur) combine with the orbitals of the surrounding atoms to create a more complex molecular orbital configuration.
For example, in phosphorus trichloride (PCl3), the three 3p orbitals of phosphorus combine with the 3p orbitals of the chlorine atoms to form three molecular orbitals. Two of these molecular orbitals are bonding, allowing phosphorus to form bonds with the three chlorine atoms. The remaining molecular orbital is non-bonding, and it can accommodate an additional pair of electrons, resulting in an expanded octet configuration.
Similarly, in sulfur dioxide (SO2), the 3p orbitals of sulfur combine with the 2p orbitals of the oxygen atoms to form molecular orbitals. Two of these molecular orbitals are bonding, allowing sulfur to form double bonds with the oxygen atoms. The remaining two molecular orbitals are non-bonding, and they can accommodate additional electrons, leading to an expanded octet configuration in sulfur dioxide.
| Compound | Central Atom | Number of Electrons in Expanded Octet |
|---|---|---|
| Phosphorus Trichloride (PCl3) | Phosphorus (P) | 10 electrons |
| Sulfur Dioxide (SO2) | Sulfur (S) | 10 electrons |
| Silicon Tetrachloride (SiCl4) | Silicon (Si) | 12 electrons |

Applications and Implications of Expanded Octets
The study of expanded octets has significant implications in various fields of chemistry and beyond. Here are some key applications and insights:
Chemical Reactivity
The ability of certain elements to accommodate expanded octets influences their chemical reactivity. Compounds with expanded octet configurations often exhibit unique reactivity patterns, leading to the formation of complex molecules with diverse applications.
For instance, the compound phosphorus trichloride (PCl3) is a highly reactive substance used in the production of pesticides and flame retardants. Its expanded octet configuration allows it to form bonds with various other elements, making it a versatile reagent in organic synthesis.
Molecular Structure and Stability
The presence of expanded octets can significantly impact the molecular structure and stability of compounds. In some cases, expanded octet configurations result in more stable molecular arrangements, leading to the formation of stronger chemical bonds.
Consider the compound silicon tetrachloride (SiCl4). Its expanded octet configuration allows silicon to form four bonds with chlorine atoms, resulting in a highly stable tetrahedral structure. This stability is crucial in various industrial applications, such as the production of silicones and high-performance materials.
Environmental Chemistry
The expanded octet phenomenon also plays a role in environmental chemistry. Certain pollutants and toxic substances can exhibit expanded octet configurations, affecting their reactivity and environmental impact.
For example, sulfur dioxide (SO2) is a common air pollutant emitted from industrial processes. Its expanded octet configuration makes it highly reactive, contributing to the formation of acid rain and other environmental issues. Understanding the behavior of expanded octets in pollutants can aid in developing effective mitigation strategies.
Future Prospects and Research Directions

The study of expanded octets opens up numerous avenues for future research and exploration. Here are some potential areas of focus:
-
Theoretical Studies: Further investigations into the theoretical underpinnings of expanded octets can provide deeper insights into the electronic structure and reactivity of these compounds. Advanced computational methods and quantum chemistry simulations can be employed to explore the behavior of expanded octets in various chemical systems.
-
Experimental Techniques: Developing new experimental techniques to study expanded octets can enhance our understanding of their behavior. Techniques such as X-ray absorption spectroscopy and electron diffraction can provide valuable data on the electronic and structural properties of compounds with expanded octet configurations.
-
Biological Applications: The expanded octet phenomenon has implications in biology and medicine. Certain biomolecules and pharmaceutical compounds may exhibit expanded octet configurations, influencing their biological activity and therapeutic potential. Exploring these aspects can lead to the development of novel drugs and treatments.
-
Materials Science: The unique electronic structure of expanded octets can be leveraged in materials science to develop innovative materials with enhanced properties. By manipulating the expanded octet configurations, researchers can design materials with improved stability, reactivity, or electronic properties, leading to advancements in electronics, energy storage, and other fields.
As our understanding of expanded octets continues to evolve, we can expect groundbreaking discoveries and applications across various scientific disciplines. The study of this fascinating phenomenon highlights the intricate nature of atomic and molecular interactions, pushing the boundaries of our knowledge and driving innovation in chemistry and beyond.
Can all elements exhibit expanded octets?
+No, not all elements can exhibit expanded octets. The ability to accommodate more than eight electrons in the valence shell is primarily observed in elements from the third row of the periodic table and beyond, such as phosphorus, sulfur, and silicon. These elements have the necessary valence orbitals to form expanded octet configurations under specific conditions.
Are expanded octets stable configurations?
+The stability of expanded octet configurations depends on the specific compound and its molecular structure. In some cases, expanded octets lead to more stable molecular arrangements, as seen in silicon tetrachloride (SiCl4). However, in other compounds, the expanded octet configuration may be less stable and can result in increased reactivity.
What are the implications of expanded octets in environmental chemistry?
+The expanded octet phenomenon has significant implications in environmental chemistry. Pollutants and toxic substances with expanded octet configurations can exhibit increased reactivity, leading to the formation of harmful byproducts. For example, sulfur dioxide (SO2), with its expanded octet, contributes to acid rain and air pollution. Understanding the behavior of expanded octets in pollutants can aid in developing strategies to mitigate their environmental impact.



