Unveiling 5 Lone Electron Pair Secrets
In the intricate world of molecular chemistry, lone electron pairs play a pivotal role, influencing the behavior and properties of various compounds. These unshared electrons, often referred to as non-bonding electrons or lone pairs, have a significant impact on molecular geometry, reactivity, and even the physical characteristics of substances. This article aims to delve into the secrets of lone electron pairs, shedding light on their significance and offering an in-depth understanding of their behavior.
1. Understanding Lone Electron Pairs

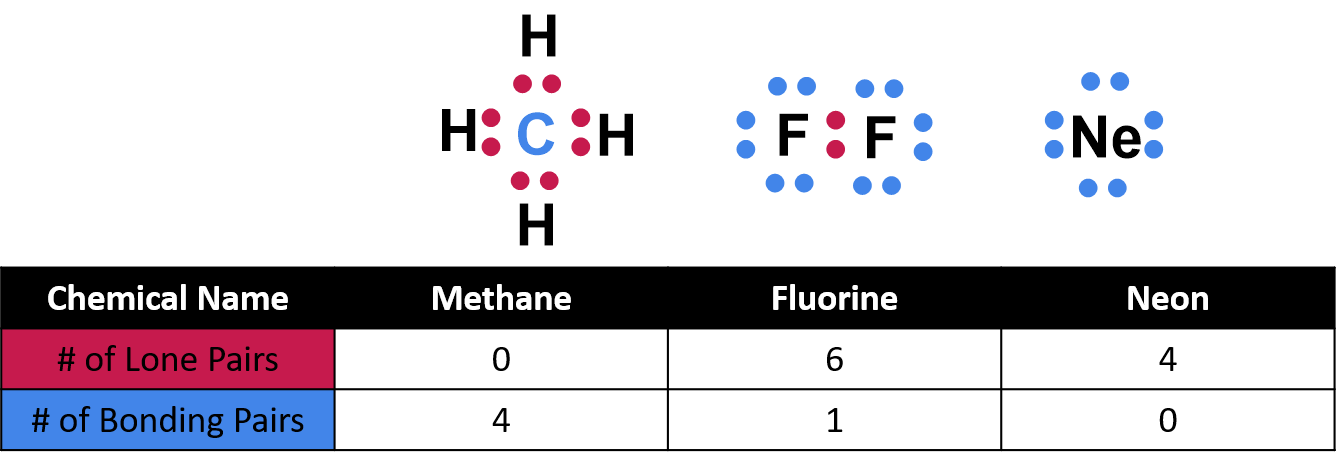
Lone electron pairs are pairs of electrons that are not involved in bonding with other atoms. Instead, they reside in the outermost valence shell of an atom, occupying their own orbital. These electrons are often depicted as two dots surrounding the atomic symbol, a visual representation of their existence.
The concept of lone electron pairs is crucial in VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts molecular geometry based on the repulsion between electron pairs. According to this theory, electron pairs, whether bonding or non-bonding, strive to maximize their distance from one another to minimize repulsive forces.
Real-World Example: Water Molecule
Consider the water molecule (H2O), a fundamental compound in chemistry. The oxygen atom in water possesses two lone electron pairs in addition to the two covalent bonds formed with hydrogen atoms. These lone pairs are responsible for the bent shape of the water molecule, with an angle of approximately 104.5 degrees between the hydrogen atoms.
Visual Representation
A visual depiction of the water molecule can be represented as follows: O (with two lines representing the bonds to hydrogen and two dots representing the lone pairs). This visual aids in understanding the electron distribution and molecular geometry.
2. Impact on Molecular Geometry

The presence of lone electron pairs significantly influences the spatial arrangement of atoms in a molecule. In VSEPR theory, the electron pairs around a central atom are considered as regions of electron density. These regions repel each other, leading to specific molecular geometries.
For instance, in the case of ammonia (NH3), the nitrogen atom has one lone pair and three covalent bonds with hydrogen atoms. This lone pair exerts a stronger repulsive force than the bonding pairs, resulting in a distorted tetrahedral shape with a bond angle of around 107 degrees.
| Molecule | Geometry | Bond Angle |
|---|---|---|
| Ammonia (NH3) | Distorted Tetrahedral | 107° |
| Water (H2O) | Bent | 104.5° |

Application in Real-World Scenarios
Understanding the impact of lone electron pairs on molecular geometry is crucial in various scientific and industrial applications. For instance, in drug design, knowledge of molecular geometry helps in creating molecules that can effectively interact with biological targets.
3. Reactivity and Lone Electron Pairs
Lone electron pairs also play a significant role in the reactivity of compounds. These unshared electrons can participate in various chemical reactions, making the compound more reactive.
For example, in the case of the hydroxyl radical (OH•), the unpaired electron is highly reactive and can readily participate in radical reactions. This makes the hydroxyl radical a powerful oxidizing agent, often used in environmental chemistry to break down pollutants.
Real-World Impact
The reactivity of lone electron pairs has practical implications in fields such as environmental science and organic chemistry. Understanding the behavior of these electron pairs can lead to the development of more efficient and sustainable chemical processes.
4. Lone Electron Pairs and Physical Properties
The presence of lone electron pairs can also influence the physical properties of compounds. These electrons can contribute to the polarity of molecules, affecting their solubility, boiling points, and other physical characteristics.
Take the case of hydrogen fluoride (HF) and hydrogen chloride (HCl). While both molecules have similar covalent bonds, the presence of lone electron pairs on the fluorine atom in HF makes it significantly more polar than HCl. This increased polarity leads to a higher boiling point and greater solubility in water for hydrogen fluoride.
| Compound | Boiling Point (°C) | Solubility in Water |
|---|---|---|
| Hydrogen Fluoride (HF) | -83.6 | Soluble |
| Hydrogen Chloride (HCl) | -85.1 | Soluble |
Industry Applications
Knowledge of how lone electron pairs influence physical properties is vital in industries such as pharmaceuticals and materials science. It allows for the design of compounds with specific properties, ensuring their effectiveness and stability.
5. Lone Electron Pairs and Intermolecular Forces

Lone electron pairs can also contribute to intermolecular forces, the attractive forces between molecules. These forces are crucial in determining the physical state and behavior of substances.
For instance, in the case of hydrogen bonding, lone electron pairs on highly electronegative atoms like oxygen and nitrogen can form hydrogen bonds with hydrogen atoms from other molecules. This intermolecular force is particularly strong and is responsible for the unique properties of water, such as its high boiling point and surface tension.
Real-World Significance
Understanding the role of lone electron pairs in intermolecular forces is essential in fields like biochemistry and environmental science. It provides insights into the behavior of biological molecules and the impact of pollutants on ecosystems.
Conclusion
Lone electron pairs, though seemingly insignificant, wield a significant influence over the behavior and properties of compounds. From shaping molecular geometry to impacting reactivity and physical characteristics, these unshared electrons play a vital role in the chemical world. Unveiling the secrets of lone electron pairs provides a deeper understanding of the fundamental principles of chemistry and their practical applications across various industries.
How do lone electron pairs affect molecular shape?
+Lone electron pairs exert a stronger repulsive force than bonding pairs, leading to specific molecular geometries. They contribute to the overall electron density around a central atom, influencing the spatial arrangement of atoms.
Can lone electron pairs participate in chemical reactions?
+Yes, lone electron pairs can be highly reactive and participate in various chemical reactions. They can act as nucleophiles or bases, contributing to the reactivity of the compound.
How do lone electron pairs impact the polarity of molecules?
+Lone electron pairs can make a molecule more polar by creating an electron-rich region. This increased polarity can lead to differences in physical properties, such as solubility and boiling points.