The Ultimate Guide: 3 H2O Electron Geometry Tips
Welcome to the ultimate guide on understanding and mastering the electron geometry of H2O, one of the most fundamental concepts in chemistry. In this comprehensive article, we will delve deep into the world of molecular geometry, specifically focusing on the fascinating molecule, water. By the end of this journey, you will have a solid grasp of the principles and applications of electron geometry, along with some expert tips to enhance your understanding.
Unveiling the Secrets of H2O Electron Geometry

Water, with its chemical formula H2O, is a simple yet incredibly important molecule. Its electron geometry holds the key to understanding its unique properties and behavior. Let’s embark on a journey to explore the intricacies of this fascinating subject.
In the realm of chemistry, electron geometry is a fundamental concept that describes the spatial arrangement of electrons around a central atom in a molecule. It provides valuable insights into the shape, bonding, and properties of molecules. When it comes to water, understanding its electron geometry is crucial for comprehending its role in various chemical processes and its significance in the natural world.
The Building Blocks: Understanding Electron Geometry
Electron geometry is a fundamental concept in chemistry, providing a framework for understanding the arrangement of electrons in molecules. It involves studying the distribution of electrons, particularly the valence electrons, around a central atom. These valence electrons play a critical role in determining the molecular shape and the nature of chemical bonds.
At the heart of electron geometry is the idea of electron domains. Electron domains represent regions in space where electrons are likely to be found. These domains can be either bonding domains, which participate in forming chemical bonds, or non-bonding domains, often referred to as lone pairs, which do not directly contribute to bonding but still influence the overall molecular shape.
By considering the number and arrangement of electron domains around a central atom, chemists can predict the shape and geometry of molecules. This understanding is crucial for predicting molecular properties, reactivity, and even biological activity. Electron geometry serves as a fundamental tool in the study of molecular structure and behavior.
| Electron Geometry Concept | Description |
|---|---|
| Electron Domains | Regions in space occupied by valence electrons, either bonding or non-bonding. |
| Bonding Domains | Electron domains involved in forming chemical bonds. |
| Non-Bonding Domains (Lone Pairs) | Electron domains that do not participate in bonding but affect molecular shape. |

In the case of H2O, water, the electron geometry is particularly intriguing. Water consists of two hydrogen atoms (H) covalently bonded to one oxygen atom (O). This simple molecular structure belies its complex behavior and significance in various scientific disciplines.
The Magic of H2O: Exploring its Electron Geometry
Water, with its chemical formula H2O, presents a fascinating case study in electron geometry. The electron geometry of water is a testament to the intricate balance between bonding and non-bonding electron domains. Let’s explore the specific electron geometry of H2O and its implications.
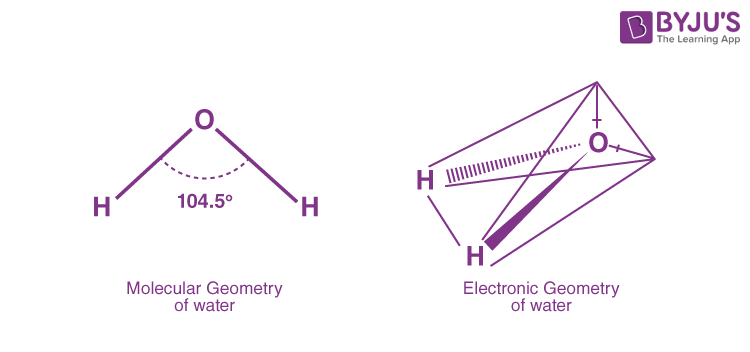
The central oxygen atom in water has six valence electrons. These electrons are distributed in a specific manner to achieve stability. Oxygen forms two covalent bonds with the hydrogen atoms, each contributing one electron to form a shared electron pair. This results in a linear arrangement of the two hydrogen atoms, with the oxygen atom at the center.
However, the story doesn't end there. The remaining two valence electrons of oxygen occupy non-bonding domains, forming lone pairs. These lone pairs exert a repulsive force, pushing the bonding electron pairs closer together. This unique arrangement gives water its distinctive V-shaped geometry, with an angle of approximately 104.5 degrees between the hydrogen atoms.
The electron geometry of water has far-reaching consequences. It influences the molecule's polarity, making water a polar molecule with a slightly negative charge on the oxygen atom and a slightly positive charge on the hydrogen atoms. This polarity is vital for water's role as a universal solvent and its ability to form hydrogen bonds, which are crucial for life's processes.
Understanding the electron geometry of water provides a foundation for exploring more complex molecular structures and their behavior. It highlights the interplay between bonding and non-bonding electron domains and how this affects molecular shape and properties. By studying the electron geometry of H2O, chemists can gain insights into the fundamental principles that govern the behavior of molecules.
Mastering Electron Geometry: Expert Tips and Insights
Now that we have a solid understanding of the fundamentals, let’s dive into some expert tips and insights to take your knowledge of electron geometry to the next level.
Tip 1: Visualize with Lewis Structures
Lewis structures, also known as electron dot structures, are a powerful tool for visualizing electron geometry. These structures provide a visual representation of the distribution of electrons in a molecule. By drawing Lewis structures for different molecules, you can easily identify the number of bonding and non-bonding electron domains, which in turn helps determine the overall electron geometry.
Let's take a look at the Lewis structure of water (H2O) as an example:
O:·H:·:H
In this structure, the central oxygen atom is surrounded by two bonding domains (the two covalent bonds with hydrogen) and two non-bonding domains (the lone pairs). This visualization makes it clear that water has a tetrahedral electron geometry, with four electron domains surrounding the central atom.
Tip 2: Understand Hybridization
Hybridization is a concept that explains how atomic orbitals combine to form new hybrid orbitals, which are essential for understanding molecular geometry. In the case of water, the central oxygen atom undergoes sp3 hybridization. This means that the 2s and 2p orbitals of oxygen mix to form four equivalent sp3 hybrid orbitals.
These hybrid orbitals then overlap with the 1s orbitals of the hydrogen atoms to form the covalent bonds in water. Understanding hybridization not only helps in determining the molecular geometry but also provides insights into the nature of chemical bonds and the stability of molecules.
Tip 3: Explore VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting molecular geometry based on the number of electron domains around a central atom. VSEPR theory suggests that electron domains will arrange themselves in space to minimize repulsions and achieve the most stable geometry.
For water, VSEPR theory predicts a tetrahedral electron geometry. This means that the four electron domains (two bonding domains and two non-bonding domains) arrange themselves in a tetrahedral shape around the central oxygen atom. This theory provides a systematic approach to understanding the electron geometry of various molecules, including H2O.
Conclusion: Unlocking the Power of Electron Geometry
Electron geometry is a powerful concept that unlocks the secrets of molecular structure and behavior. By understanding the electron geometry of molecules like H2O, we gain insights into their unique properties, reactivity, and significance in various scientific disciplines.
Throughout this guide, we have explored the fundamentals of electron geometry, delved into the specifics of water's electron geometry, and provided expert tips to enhance your understanding. Whether you are a student, a researcher, or simply curious about the world of chemistry, mastering electron geometry is a valuable skill that opens doors to a deeper understanding of the molecular world.
So, embrace the power of electron geometry, continue exploring, and unlock the wonders of molecular science.
How does electron geometry affect molecular behavior?
+Electron geometry plays a crucial role in determining molecular behavior. It influences the shape and polarity of molecules, which in turn affects their physical and chemical properties. For example, the electron geometry of water gives it a bent shape, making it polar and allowing it to form hydrogen bonds, a vital property for its role in various biological processes.
What are the applications of understanding electron geometry in chemistry?
+Understanding electron geometry has numerous applications in chemistry. It helps predict molecular shapes, bonding patterns, and reactivity. It is essential for designing new molecules, understanding biological molecules, and even in fields like materials science and environmental chemistry. Electron geometry provides a foundation for studying the behavior of molecules and their interactions.
Can electron geometry be applied to more complex molecules?
+Absolutely! Electron geometry principles can be applied to a wide range of molecules, from simple diatomic molecules to complex organic compounds. While the concepts may become more intricate for larger molecules, the fundamental principles of electron domains, hybridization, and VSEPR theory remain applicable. Understanding electron geometry is a powerful tool for studying and predicting the behavior of diverse molecular structures.



